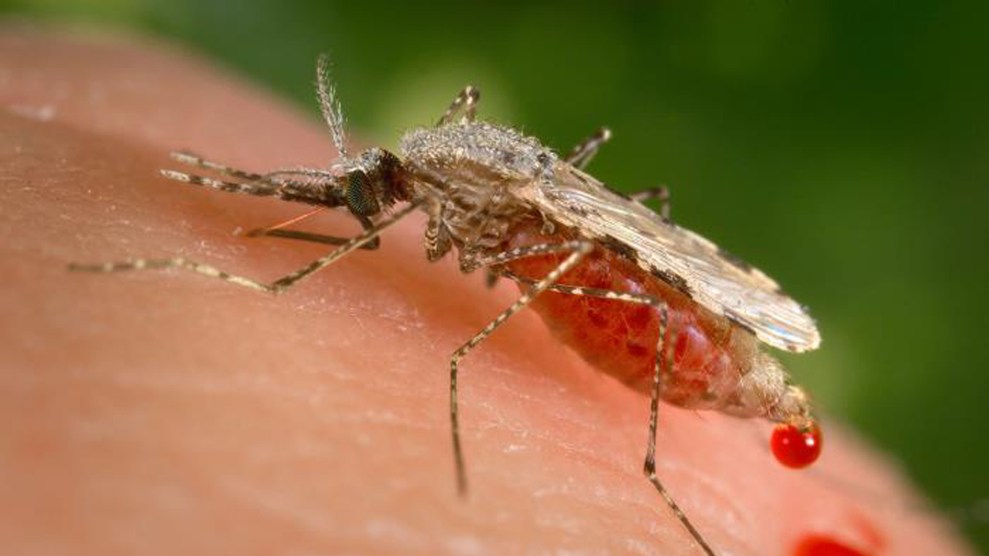
The capabilities of “gene drive” are thrilling—and also terrifying.
A gene drive currently in development could render Anopheles stephensi mosquitoes unable to spread malaria.James Gathany/AP
Dengue, which infects up to 100 million people worldwide each year, is spread largely by Aedes aegypti mosquitoes, which thrive
along our Gulf Coast and also are capable of transmitting the related
viruses Zika, chikungunya, and yellow fever. Of the millions infected,
roughly 500,000 dengue victims develop an excruciatingly painful
“break-bone fever”—according to Laurie Garrett’s The Coming Plague, “dengue” derives from the Swahili phrase ki denga pepo, “it is a sudden overtaking by a spirit”—and tens of thousands die.
West Nile virus, spread by Culex mosquitoes, has killed
more than 2,000 Americans since 1999, primarily in California,
Colorado, and Texas. Our latest headache, Zika, produces ghastly brain
defects in the infants of infected mothers and neurological symptoms in
some adults. Puerto Rico has been ravaged by more than 35,000 mosquito-borne Zika cases since 2015, not to mention periodic dengue outbreaks that afflict tens of thousands of people.
What if we could make all of this go away?
We do, in fact, have a weapon that could end the mosquito’s reign of
terror. It’s called “gene drive,” and its implications are thrilling—and
also kind of terrifying.
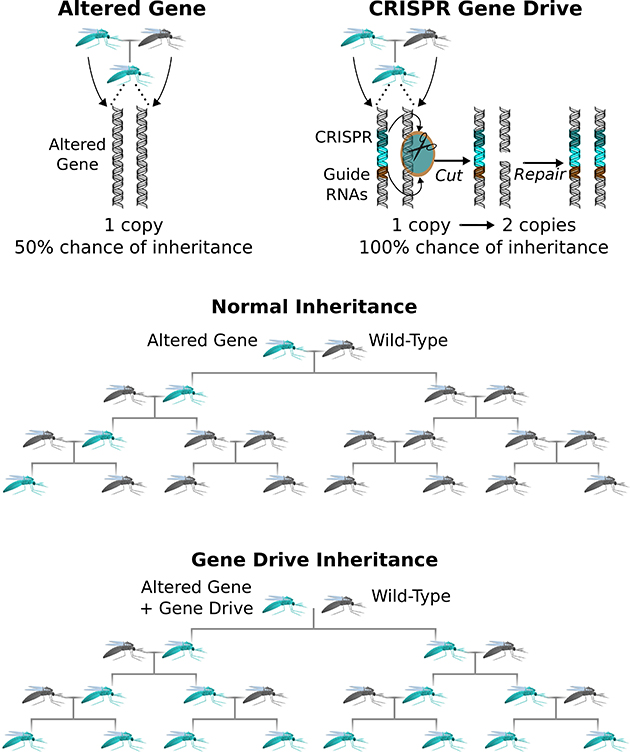
Evolution is a numbers game. Say you were to engineer a lab-modified
gene into an animal embryo. By the rules of inheritance, that anomaly
would be passed along to roughly half the creature’s offspring. Assuming
the new gene didn’t offer any survival advantage (or disadvantage), it
would be inherited by about a quarter of the subsequent generation and
then an eighth and a sixteenth, and so on—until it became the genetic
equivalent of radio static.
Gene drive upends that calculus. Lab-tested so far in yeast, fruit
flies, and mosquitoes, this powerful new technique guarantees that a
modified genetic trait is inherited by virtually all a creature’s
offspring and all their offspring. After a while, every individual in the population carries the modification.
If used with care, gene drive could save millions of lives and
billions of dollars. It could reduce pesticide use, help weed out nasty
invasive species, and prevent tremendous human suffering. Then again, it
could have unintended social and ecological consequences—or be hijacked
for malevolent purposes.
The concept of a gene drive was first described in a 2003 paper
by Austin Burt, a respected British geneticist. Burt was inspired by
the naturally occurring “selfish” genes that are able to copy themselves
around a genome. Harnessing this ability and improving on it, he
realized, could allow scientists to engineer natural populations, with
an eye, for instance, toward preventing the spread of malaria.
Burt’s insight wasn’t practical, though, prior to the fairly recent invention of a breakthrough technique called CRISPR-Cas9 gene editing.
With this innovation, a scientist uses customized ribonucleic acid
(RNA) guide sequences to deliver a molecular scissors (an enzyme called
Cas9) to a precise spot on a chromosome. The enzyme snips the double
helix, prompting the cell’s DNA-repair machinery to kick in and patch
things up—and in the process replacing the wild-type gene at that
location with a lab-engineered DNA sequence. (Here’s one simple diagram.)
One spring day in 2013, about a decade after Burt’s paper appeared, a
30-year-old researcher named Kevin Esvelt was out walking in the
Boston-area greenbelt known as the Emerald Necklace, pondering his next
move. Esvelt, a post-doctoral fellow working with the renowned Harvard
geneticist George Church, had ruled out working on the development of
new CRISPR techniques. “The field had become so crowded,” he recalls via
email, “it seemed likely almost anything I tried would be pursued by at
least three other labs.”

MIT Media Lab
And that’s when it hit him: Scientists had been putting the CRISPR
tools into their target cells as separate pieces. What if you introduced
them into the embryos as a single, heritable element? Those creatures
and their descendants—all of them—would retain the gene-editing ability
in their DNA. The system would be self-propagating. In short, you could
rig nature’s game so your gene would win every time!
He took his ideas and concerns to his mentor, George Church. A
scientist’s usual first instinct is to test an exciting hypothesis right
away to see whether it’s viable, and then be the first to press with a
blockbuster paper. This felt different. “We decided not to immediately
test it in the lab—not because we couldn’t do it safely, but because we
felt that no technology like this should be developed behind closed
doors,” Esvelt says. “The question was whether it was safe to tell the
world.” At Church’s urging, they brought on Jeantine Lunshof, an
ethicist, and Ken Oye, a social scientist and policy expert: “Ken’s
first words after I described the probable capabilities were not
publishable.”
The researchers determined that their best course was to go public
before doing any experiments. They solicited feedback from fellow
molecular biologists, ecologists, risk analysts, public policy and
national security experts, and representatives of environmental
nonprofits. Only then, in July 2014, did they publish a pair of papers
on gene drive’s uses and policy implications.
This summer, a group of researchers that consults for the federal
government was tasked with analyzing the technique’s potential
risks—including the possibility that it could be used for biowarfare.
“The range of nefarious possibilities based on genetically engineered
microorganisms is already vast,” Steven Block, an expert in bioterror
defense at Stanford University, told me in an email. “The right question
to ask is whether a hypothetical gene-drive-based bioweapon, which is
based on multicellular organisms, would afford any specific advantages
over something based on microorganisms. Would it be more powerful?
Cheaper? Easier to construct? Would it be more accessible to an
adversary? Would it afford any special ‘desirable’ properties as a
weapon, from either a strategic or tactical perspective? I’d argue that,
at least for the time being, gene drive seems to have done little to
change the lay of the land.”
Accidents, mistakes, and unsanctioned releases are a separate
concern. But Esvelt and his peers realized, to their great relief, that
gene drives can be overwritten; they spread slowly enough through a
population and are easy enough to detect, Esvelt says, that researchers
should be able to stop a rogue drive using something called an
“immunizing reversal drive” that can cut up the engineered sequence and
restore the original genes. (He and Church have demonstrated
the reversal process in yeast.) In any case, he says, it would be
“difficult to imagine any possible combination of side-effects worse
than a disease like malaria.”
Over the past couple of years,
several labs have proved that gene drives work as hypothesized. The next
step is to convince society they can be tested safely. Each drive is
different, so potential risks and benefits have to be weighed on a
case-by-case basis. But one big-picture problem is that wild creatures
don’t respect human boundaries. A drive could easily scamper or fly or
tunnel across borders and into areas where it hasn’t been sanctioned by
local authorities. And that, Esvelt says, could trigger “international
disputes or even wars.”
In his new position as head of the Sculpting Evolution group at the Massachusetts Institute of Technology’s Media Lab, Esvelt is working on gene-drive variations
that can limit the spread of the engineered genes to a given number of
generations. But diplomacy will be needed regardless. “For malaria, the
case for an international agreement is obvious,” Esvelt says. Ditto the
New World screwworm, whose “existence in the wild is an atrocity from an
animal welfare perspective—it literally exists by eating higher mammals
alive, causing excruciating agony.”
In 2015, Austin Burt and his collaborators unveiled a gene drive designed to decimate populations of the African malaria mosquito Anopheles gambiae
by rendering all female offspring sterile, although for statistical
reasons, it is “quite implausible” for a gene drive system to completely
wipe out a problematic species, Esvelt says. “Suppress a population,
sure. Locally eliminate, possibly. But extinction? Not by itself.”
Anthony James, a geneticist at the University of California-Irvine,
opted to target the disease directly. In 2015, he and his colleagues
lab-tested a drive that enlists a pair of synthetic antibodies to disable malaria in the gut of the South Asian mosquito Anopheles stephensi.
The dual attack—which targets two distinct phases of the parasite’s
life cycle—should be all but impossible for the organism to overcome. In
the highly unlikely event that these antibodies were to get into
another insect species, they shouldn’t cause any problems. And because
the mosquito population remains intact, their predators won’t lack for
food.
James says his malaria drive will be ready for field tests within two
years—either in huge outdoor cages or within a naturally confined
environment such as an island. But is humanity ready to allow it? “It’s
all new stuff. This is the problem. There’s no pathway,” he says.
Securing permission to move forward with testing will depend entirely on
the local mood and regulatory situation. As for deploying gene drive on
a species-wide scale? Esvelt is skeptical that nations would accept
wild releases without constraints in place that would limit their scope.
One way or the other, something has to change on the mosquito front.
Conventional control methods—monitoring and education, poisons,
door-to-door efforts to eliminate standing water—aren’t working. Poor
countries in particular lack the resources to keep the bugs at bay, and
because insects and microorganisms evolve so rapidly, our chemical
weapons are rapidly losing their effectiveness. According to Bill
Reisen, a retired UC-Davis mosquito expert, California mosquitoes can
now tolerate compounds from three major families of insecticides that
were once used to kill them: “The opportunities for control are becoming
progressively limited.” The Centers for Disease Control and Prevention reports that Plasmodium falciparum, the world’s deadliest malaria parasite, has developed resistance to “nearly all” antimalarial drugs.
A Zika vaccine seems to be on the horizon, but dengue remains a frustratingly elusive target
for vaccine developers. UC-Davis geneticist Greg Lanzaro told me last
year that, were it solely up to him, he would deploy gene drive as soon
as scientifically feasible to beat back the Aedes mosquitoes
that spread these diseases. Esvelt has heard similar sentiments from
peers in several fields. “As a scientist, it’s hard to accept
nontechnical limitations, especially when we could seemingly save so
many lives if those constraints somehow magically vanished,” he says.
“But they won’t.”
One thing is for sure: “The first effort has to be an unqualified
success,” James says. “If there’s a trial and it’s a disaster—meaning it
doesn’t prevent an epidemic—the technology is going to be set back.”
Esvelt points to Jesse Gelsinger,
an 18-year-old whose death during a 1999 gene therapy trial stifled
progress in that field for a decade or more. “An accident involving a
CRISPR gene drive—which would be viewed as reckless scientists
accidentally turning an entire species into GMOs—would almost certainly
have similar effects,” he says. And in the case of malaria, the delay
“would likely result in the otherwise preventable deaths of millions of
children.”
So he’s willing to wait to get it right. Indeed, in Esvelt’s view,
gene drive is so existentially powerful that it demands a new era of
scientific transparency. If researchers don’t rethink their longtime
custom of competing behind closed doors, “we are likely to open
extremely dangerous technological boxes without even realizing it.” A
deeply collaborative approach with preregistered experiments, he says,
would help scientists identify unforeseen dangers and ensure that those
“boxes remain closed until we can develop countermeasures.” Such a
radical departure from the current culture of secrecy would require
nothing short of a sea change in the scientific community. But it might
be worth the effort. As Esvelt puts it, “The greatest potential
application of gene drive is to engineer the scientific ecosystem.”
This Technology Could Stop the World’s Deadliest Animal
Posted by Focus on Arts and Ecology on
- -
Posted in
Health and Wellness












Đăng nhận xét